Brazil approves and rolls out AstraZeneca and Sinovac vaccines
- 4 years ago
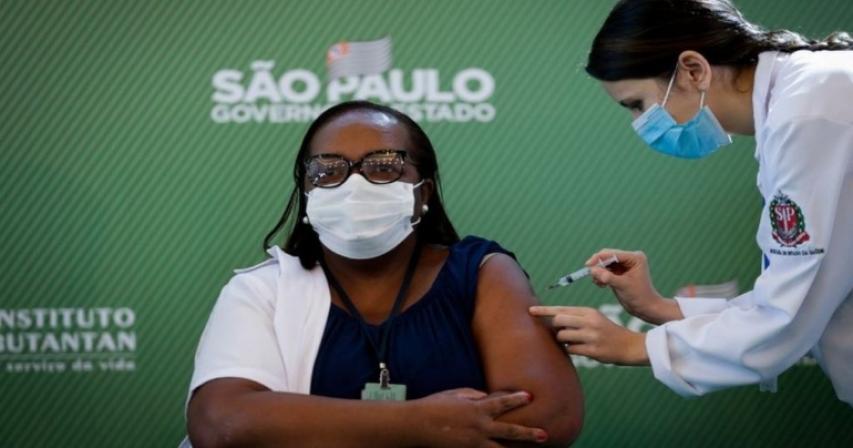
A nurse has received Brazil's first Covid-19 vaccine dose after regulators gave emergency approval to two jabs.
Regulator Anvisa gave the green light to vaccines from Oxford-AstraZeneca and China's Sinovac, doses of which will be distributed among all 27 states.
Brazil has the world's second-highest death toll from Covid-19 and cases are rising again across the country.
President Jair Bolsonaro has been heavily criticised for his handling of the pandemic.
The far-right leader has played down the pandemic from the beginning, promoted an unproven treatment for the disease and gone against measures including mask-wearing and social distancing.
The president, who caught Covid-19 last year and recovered, has said he will not take a vaccine.
Authorities reported 551 new fatalities on Sunday, the first time in six days that it had fallen short of 1,000 although this could reflect a delay in the reporting of numbers over the weekend.
In all, more than 209,000 Covid-related deaths have been recorded in Brazil, a raw total figure only exceeded by the US.
Over 8.4 million infections have been confirmed since the start of the pandemic - the third-highest tally in the world.
Health Minister Eduardo Pazuello told reporters that the national vaccination programme in the country of 211 million people would begin in earnest in the coming days. Two Brazilian biomedical centres which have been given approval to produce the jabs will be heavily involved.
About six million doses of the Sinovac-developed CoronaVac have already been produced in Brazil, while the government is waiting for shipments of the AstraZeneca vaccine from a laboratory in India.
Shortly after Anvisa's board gave emergency approval, Monica Calazans, a 54-year-old nurse in São Paulo, became the first person to be inoculated with CoronaVac.
Her vaccination was organised by the São Paulo state government, which is led by Mr Bolsonaro's main political rival, João Doria.
Comments